There’s something magical about growing crystals – whether you’re growing crystal names or growing crystal trees. No matter how many times we’ve waited for the stunning structures to appear, the surprise at what has grown never disappoints. This crystal tree demonstration is no exception.
The surprisingly simple experiment is the perfect addition to our top-selling Super Cool Science Kit!
This post contains Amazon affiliate links.

Getting Ready to Grow Crystals
The hardest part of growing crystals, besides waiting, is finding the key ingredient, Mrs. Stewart’s Bluing. (Other brands won’t work for this demo.)
Bluing is a laundry additive used to brighten whites and sometimes can be found in the laundry section of the grocery store. I avoided the frustration of hunting it down and simply ordered it from Amazon here.
Once my bluing arrived, I gathered my materials:

- Cardboard (the back of a paper pad works best)
- Dish (large enough to hold your tree, with room for the crystal to grow)
- Water
- Table salt
- Mrs. Stewart’s Bluing
- Ammonia (optional, but speeds up evaporation)
- Green food coloring (optional)
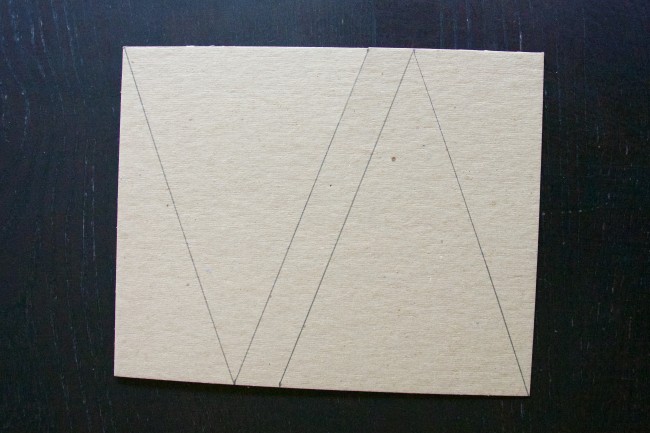
(The first time we tried this, I just eyeballed how big I wanted the tree to be. It ended up being 6 inches wide at the base and 8 inches tall. I later learned that a tree this height is way too tall for growing crystals.)
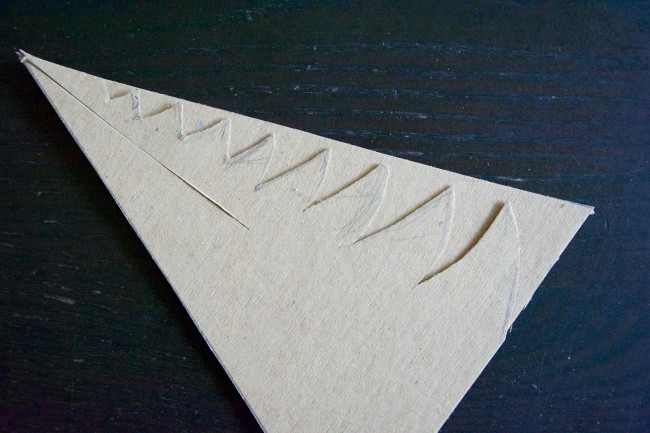
After I cut out the triangles, I cut out the branches and used the first tree to trace the branches on the second tree.
Making the Magic Crystal Solution
- 4 tablespoons warm water
- 4 tablespoons table salt
- 4 tablespoons Mrs. Stewart’s Bluing
- 2 tablespoons ammonia
Several days later, the trees were covered in delicate needle-like crystals. My daughter thought they looked a lot like cauliflower.
My kiddos were so curious what the growing crystals felt like and since the white tree was getting pretty droopy, I let them go for it.
The Science Behind It
The solution we mixed up created a suspension, a mixture that has solid particles that are large enough for sedimentation (settling out). Our solid particles settled out on the cardboard tree due to a couple of processes.
First, the liquid suspension is drawn up the cardboard tree through a process called capillary action. Capillary action is the ability of a liquid to defy gravity and flow upwards.
In narrow areas, the combination of surface tension of water and the adhesive forces between the container and the liquid help to lift the liquid. This effect can be seen when a plant like celery draws up colored water through its capillaries, when paint is drawn up between the bristles of a paint brush and when porous materials like cardboard draw up water.
The next process is evaporation, where liquid molecules escape from the solution as gas molecules.
Ammonia evaporates much quicker than water and we added it to the solution to speed up the evaporation process. Once the solution travelled up the cardboard and evaporated, the next process could occur.
The final process, crystallization, occurred as the water and ammonia molecules leave the solution through evaporation. The bluing and salt that remain can no longer be supported in the remaining solution and begin to crystalize.
Mrs. Stewart’s Bluing is a colloidal suspension of extremely minute particles of blue powder (Ferric Hexacyanoferrate). These minute particles act as nuclei, or seeds, for crystallization to take place. This is similar to the process that occurs in cloud formation.
The Super Cool Science Kit
For more science activities kids love, grab our Super Cool Science Kit packed with 30 jaw-dropping experiments and a ready to go science journal.















30 Comments