These kids’ science experiments use one egg and a few simple kitchen items for some seriously egg-citing results. The three sequential egg experiments are super simple to complete but are jam-packed with learning. Kids will LOVE watching their egg “magically” transform!
Follow the simple step-by-step below and then grab 30 more easy-to-follow science experiments kids will beg to repeat (plus a no prep science journal to keep track of their results!) in our shop!
Getting Ready
To prep for the science experiments, I grabbed a few supplies:
- Raw eggs (we did multiples, in case one broke when being handled)
- Vinegar
- Corn syrup
- Water
- Food dye
- Glasses or Jars (lids can help keep the vinegar smell in check)
Disappearing Egg Shell

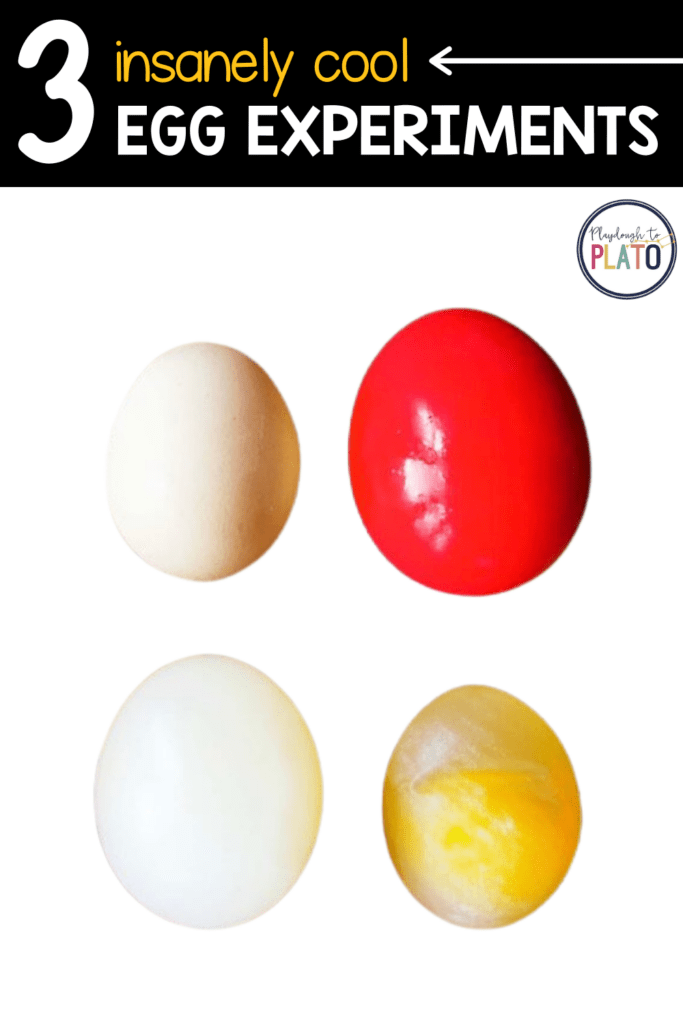
For the first experiment, I handed my daughter a raw egg and asked her what she thought would happen if we placed it in vinegar. “It would get dyed,” she replied, remembering how last spring we used vinegar and food coloring to dye eggs.
I had her label 2 cups, one for water and one for vinegar. I carefully placed an egg in each and my daughter covered one with vinegar and one with water (the control). Right away, there was a reaction in the vinegar glass. Tiny bubbles formed all over the egg and caused the egg to rise to the surface. Both my 2.5 and 5 year-old were fascinated. Within a few hours, you could see the vinegar eating away the egg shell.
We left the eggs overnight and in the morning, almost all the shell was eaten away and the top of the vinegar was foamy.

We used both brown and white eggs and the brown eggs had more visible results. You could see the remains of brown shell floating in the foam and it was much easier for my kids to see that the shell was being broken down by the vinegar.
Next, I carefully rinsed the vinegar egg under a gentle stream of water, rubbing away any broken down shell with my fingers. There was still a thin layer of shell on the egg so we placed the egg back in the glass, added fresh vinegar and let the eggs sit overnight.
On the third day, the shell was completely gone and my kids could not wait to get their hands on the rubbery, naked egg. I handed A the egg and, while she gently squeezed and rolled the egg around, I asked her what she noticed. She held the translucent egg up to the sunlight to see the yoke and the thick white strand, called the chalazae, that anchors the yolk and holds it in place within the egg. She observed that it didn’t have a shell, was “squeezy”, stunk and was bigger. I explained that the acid in the vinegar broke down the calcium carbonate shell, producing the tiny carbon dioxide gas bubbles we saw. The egg got bigger because the membrane is semi-permeable and because of a process called osmosis.
To illustrate how the membrane allows gases or liquids to pass through it, I had A blow through the fabric on her sleeve. “Did you feel your breath?” I asked. When she nodded yes, I explained that her shirt has tiny holes that allow her breath (a gas) to pass through. The egg membrane is like that, too – it allows some things to pass through it.
I explained that osmosis is the movement of a liquid, like water, across a membrane. I explained that membranes like to be balanced on both sides. The vinegar solution is mostly water with only a little vinegar in it, while inside the membrane is protein with a little water. So the water molecules travel from the vinegar into the egg to try to balance the concentrations, and the egg expands.
I then told A she could try to bounce the egg a few inches off the table. She was expecting an explosive mess as you can see, but the egg did indeed bounce. Awesome!
The Shrinking Egg
After a few more bounces, we moved on to the second part of the experiment: making the naked egg shrink. For this part, A simply filled a glass with corn syrup and placed in the shell-less egg. The egg floated to the surface but, don’t worry, it didn’t affect the experiment.

I explained to A that corn syrup is very sugary with a little water dissolved in it. I asked A what she thought would happen to the egg, reminding her how the membrane likes to stay balanced on both sides and will move water to do so. “The egg will get smaller,” she replied.
When we came back the next day, we could see the egg had shrunk and the corn syrup had a thinner layer on top. This was where the water left the egg, causing the shrinking.

We very carefully removed the shriveled looking egg from the corn syrup and rinsed the stickiness away under a gentle stream of water. Again, A rolled and squeezed the deflated egg in her hands. “You can feel the yolk,” she said excitedly. The yolk felt surprisingly hard.
During this experiment I left the control egg in the water and another in the vinegar. I got both these eggs out for A to examine side-by-side and observe the different changes that occurred. We could have left the shrunken egg in the corn syrup for even more dramatic results but A got the idea of osmosis so we moved on.
The Expanding Colored Egg
For the last part of the experiment, A added several drops of food coloring to a glass of water and plopped in the deflated egg.
The following morning, the egg was once again round, bouncy, and bright pink! I asked A if we should see how high the egg can be dropped and still bounce. She was reluctant to risk her shiny pink egg but eventually gave in. 
We examined the popped egg and A noticed the whites of the egg seemed watery and pink! “The pink water DID go into the egg!” she exclaimed.
Seriously egg-citing!
30 More Egg-citing Science Experiments
Grab 30 easy-to-follow science experiments kids will beg to repeat (plus a no prep science journal to keep track of their results!) in our shop!













Awesome! That’s a lot of science from just one egg!
if I just leave the egg in the vinegar for 10 days will it last in it’s “rubberized” state until we take it out? Or will it go “bad” before then?
I think it would still be in it’s rubbery, bouncy state but I wouldn’t want it to burst anywhere near me. If you chose to keep it in vinegar that long, I would keep it in the fridge. It is a raw egg after all!
Thank you so much ! I had a wonderful time with my 5 years old son. And I’ve also learned what osmosis was… We don’t have corn syrup in France but it has perfectly worked with agave syrup.
Just to be clear, when you said the eggs in vinegar and water, is the egg raw or do you boil it first?
Thank so much for the detailed explanation.
I just saw this and love it. Now I’m all set for science in kindergarten next week. I would like to add the diffusion, and osmosis is part of the grade 8 curriculum in Ontario. I would use these and have older students explain what and why these things happened!
Thank you for this! Our preschoolers at my school loved this experiment! They went home telling their parents all about it. Can’t wait to try it with my own kiddos! Great job and thank you for posting!
I love how you explained osmosis and semi-permeable membranes to your daughter. Simple, but scientifically accurate.
Is there anything I can use instead of corn syrup? We are half way through and I don’t have any!!
Hmmm… perhaps maple syrup? Whatever you choose it would have to have a low concentration of water and high concentration of sugar. The syrup might work but it seems too watery to me and expensive! You could always wait and leave the egg in the vinegar for a day while you get corn syrup.
Honey might work as well. 🙂 It’s high in sugar and is a higher density to maple syrup.
This experiment was fun, but oh what a mess!